
Regulatory Information Management (RIM) systems are becoming more prevalent in medical technology companies of all sizes. Yet many regulatory teams still rely on spreadsheets and software designed for other purposes, such as quality systems or pharmaceutical regulatory applications. When your team is ready for a medical device RIM system, what information and arguments can you use to obtain the budget and executive buy-in you will need?
In this article, we discuss the benefits of a RIM system that can be used in calculating and estimating ROI, along with examples of results achieved by Rimsys customers.
Improved efficiency
Arguably the greatest benefit to implementing a RIM system is the increased process efficiencies it brings, but this benefit is often the most difficult to quantify. It is not difficult to imagine that moving from spreadsheets and manual processes to a dedicated regulatory information management system will improve efficiency, but how do you measure this?
- Eliminate “non-value add” work
Identify the processes on which your RA team spends the most “non-value add” time. How much time does it take for them to determine all of the countries in which a product is approved for sale? What registrations are expiring this year? What GSPRs need to be updated because a standard has changed? For many medical device manufacturers, these processes take hours, days, or even weeks, of combing through multiple data sources and verifying information. A properly implemented RIM system can be expected to provide this type of information in minutes. - Improve communication between departments
Consider how your systems and departments communicate with each other. When the product team makes a change, how quickly and seamlessly are the quality and regulatory teams notified? Do they always have the time they need to react to such changes? If the regulatory team identifies a new requirement that the quality and product teams need to be aware of - how seamlessly is that handled? A RIM system can not only identify items that need to be communicated to other teams, but can also be integrated with PLM, eQMS, and ERP systems to automate such communication. One good example of this is Rimsys’s ability to share a product’s selling status with the manufacturer’s ERP system. This ensures that a product is never sold into a market where it has not been approved. - Enforce company processes and workflows
A RIM system can help enforce your processes and ensure proper communication by managing approvals and other tasks within the system. By automating communications around process tasks, teams do not need to rely on individual emails (or remember to send those emails). RA teams don’t need to hunt through email history to confirm that they haven’t missed anything, and processes, approvals, and actions are recorded in a secure and compliant system.
Reduce the impact of RA staff turnover
A strong RIM system not only helps to reduce the risk and cost associated with staff turnover, but can also help reduce turnover in the first place! When RA staff turns over, or a new member joins the team, a RIM system will provide:
- Clear and defined processes that are standardized and built into the system.
- A central repository of product registration information, submission records, and more.
- Immediate availability of current and historical records when dealing with regulatory agencies and notified bodies.
A RIM system also speeds up the onboarding process new RA team members, which can otherwise take 6 months or more for employees to get fully up to speed on the product portfolio, in-flight and upcoming projects, and previous interactions with health authorities.
Providing your existing RA team with a well-implemented RIM system reduces the time they spend searching for information, allowing them to spend more time doing what they do best—implementing regulatory strategies and managing the regulatory affairs of the company. Your RA team will be more productive, feel more empowered, and be more likely to say in their role.
Minimize compliance risks
Medtech regulatory teams need to ensure that they are staying current with ever-changing global regulations, guidance documents, and standards. Each change needs to be evaluated for its impact on items such as existing GSPRs and pending compliance deadlines (think of the changing UDI labeling and database deadlines in many countries). RA teams are also responsible for ensuring that required reporting and submission deadlines are met for every product in every country in which they are sold.
RA teams that rely on manual processes and spreadsheets are opening their companies to a higher level of compliance risk than those using holistic RIM systems. RIM systems can automate many of the processes required to ensure regulatory compliance, including:
- Identification of GSPRs affected by a standards change.
- Notifications of pending license expirations and regulatory deadlines.
- Approval and notification tasks.
Without a central regulatory system and automated processes, required regulatory actions may be missed resulting in expired registrations that require products to be pulled from the market or audit findings resulting from information being incomplete or unavailable.
In addition, RIM systems like Rimsys are designed to be verified under 21 CFR part 11 requirements and provide quick access to data required during an audit or by a notified body or regulatory agency.
Reduced costs
Wasted time
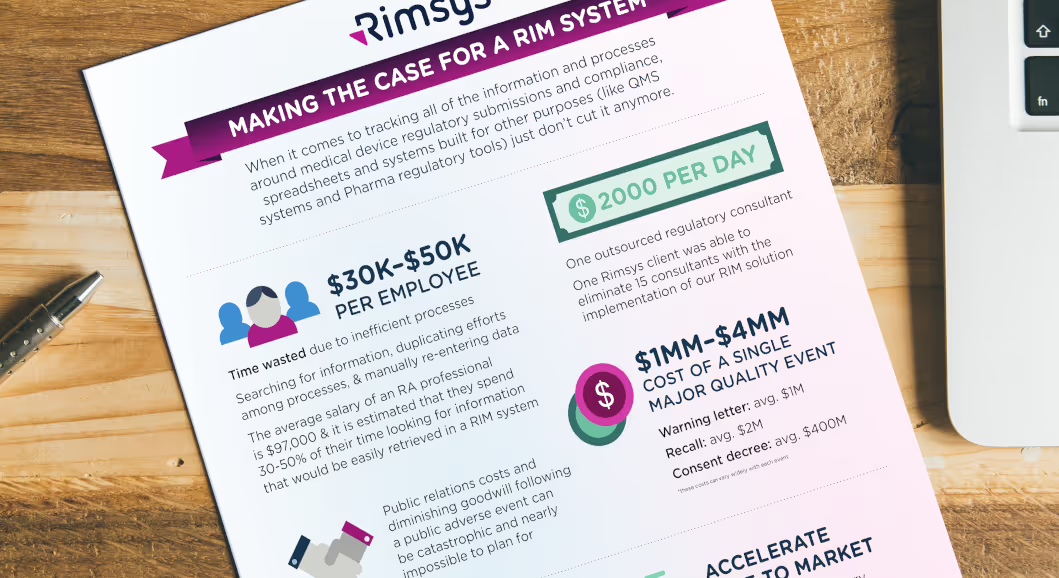
Many of the RIM advantages discussed above also lead directly to cost savings. When making the case for a RIM system in your organization, use as much specific data as possible - including average RA salary and time-savings estimates based on your team and processes. In general, though, consider that:
- The average RA professional wastes 30-50% of their time looking for information that could be easily retrieved with a RIM system.
- The average salary of an RA professional is $97,000.
- Approximately $30-$49k of each employee's salary is wasted due to inefficient processes.
In addition, a RIM system may allow you to reduce the cost of outside consultants and contracted regulatory work. Medtech regulatory consultants can charge between $150 and $300 an hour - resulting in consultant fees in the millions of dollars for many medical device manufactuers. One Rimsys customer was able to eliminate 15 consultants at the time they implemented the Rimsys RIM solution.
Cost of non-compliance
If your organization is found to be out of compliance by any regulatory agency, the cost can be extremely high. Not only must you put time and effort into becoming compliant, but you may likely face fees, penalties, higher consultant fees, and other direct costs. If a product needs to be removed from a market, and then re-approved, the costs can be significant. The largest concern for most companies, however, may be the costs associated with a well-publicized non-compliance issue (often following an adverse event or major quality issue). While difficult to quantify, if your company has faced major recalls or other public issues, use the actual lost revenue and increased cost numbers as available.
According to a McKinsey report, the average share value of a company experiencing a major quality event drops by 16.8%. The same report lists the average cost of a recall in companies surveyed at $2 million, a warning letter at $1 million, and a consent decree at $400 million (this last number is one consent decree at a single company).
Increased revenue
We believe that regulatory teams do not get enough credit for driving revenue within their organizations. A well-run regulatory team with the right tools drives:
- Increased speed to market: Regulatory teams using RIM systems complete new product submissions and registrations renewals in much less time than those without dedicated regulatory software. This means more products getting to market more quickly. Consider estimating how many weeks/months you can reduce product submission activities by and estimate additional revenue based on expected product releases in the coming year.
- Less revenue at risk from compliance issues: The potential for lost revenue can also be reduced by improving regulatory processes through a RIM system. If a product needs to be pulled from a market or experiences a serious and public regulatory event, how much revenue will your company lose in that market during the months or years it will take to recover? Medical device manufacturers reduce this risk by implementing strong regulatory systems that ensure registration renewals, ongoing reporting requirements, and updated requirements are visible and well-managed.
Real-world examples from Rimsys customers
- A leading In-Vitro diagnostic manufacturer reduced the time it took to update the 1400 GSPRs they were managing when a single standard changed by from 360 person-hours (3 regulatory professionals x 3 weeks) to 30 minutes. The time to create a GSPR table was reduced by 50% and required maintenance was reduced by 99%. (read the full case study)
- One medical device company had no communication between their PLM, eQMS, and ERP systems - causing delays in getting products registered and into new markets. They implemented Rimsys (replacing existing spreadsheets) and streamlined their product authorization process - reducing workload by 88%. It now takes just a few minutes to determine where a product is sold, versus the hours it took previously. (read the full case study here)
- BISCO, a leading global manufacturer of dental adhesives and cement, has a well-organized product registration process, but the information was difficult to share and search. Maintaining essential principle tables was also a growing concern. According to Ryan Hobson, BISCO's Global RA Manager, Rimsys allowed them to take “a process that could take a week or a week-and-a-half all told, and shortened it to a matter of minutes.” (read the full case study here)
Looking for information and data you can use to make the case for budget or leadership buy-in for a regulatory information management project? Download our RIM ROI infographic for a quick reference of all of the potential cost savings and revenue growth that can be realized with a RIM system.
Similar posts







.avif)

