Digitally create and maintain medical device essential principles
Easily organize and manage detailed safety and performance documentation for all of your products
Request a demo
As more countries adopt essential principles for objective evidence of product compliance, medtech companies are struggling to keep up. Analysts have estimated that up to 50% of products will be withdrawn from the European market as the new general safety and performance requirements (GSPR) of the MDR and IVDR regulations come online.
Rimsys streamlines the creation and management of essential principles tables, allowing you to make bulk updates, automate approval workflows, and receive notifications when standards or evidence change.
Medical device essential principles/GSPR software from Rimsys

Collaborate with your team to determine applicability, apply standards, and maintain your evidence of compliance

Connect to your document management, PLM, and quality systems to directly integrate information into your tables
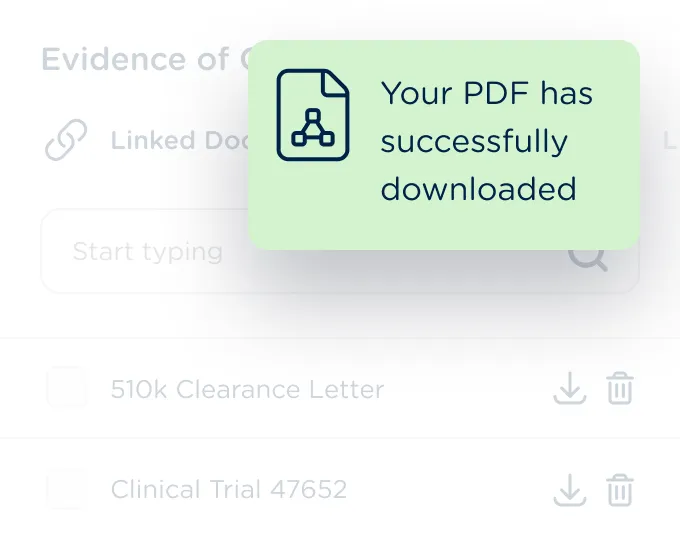
Export completed tables and supporting documentation into consolidated PDF documents for simplified submissions
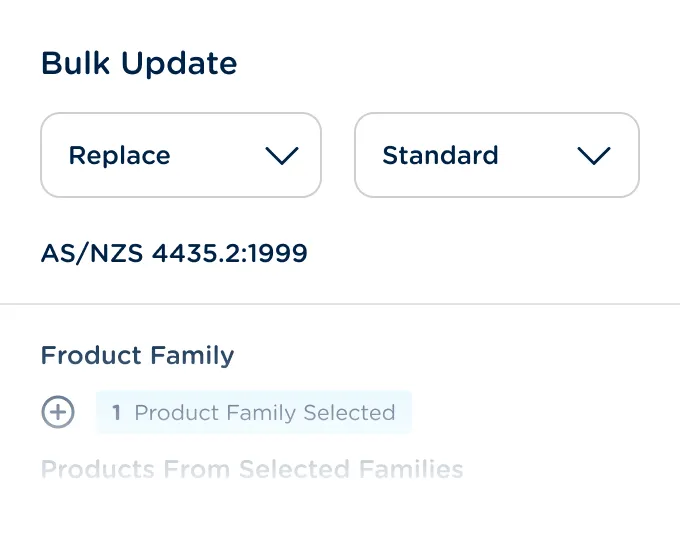
Automatically propagate record changes across multiple products and product families

Integrate automated standards management with your essential principles tables, and get notified of changes

Digital MDR, IVDR, and TGA essential principles tables can be easily customized for notified body requirements



