Proactively monitor and manage medical device standards
Track any medical device standard and assess the impact of changes on your product portfolio
Request a demo
Maintaining international standards compliance can help ease market entrance, but it can come at a high cost. Keeping track of hundreds of standards, identifying changes, and understanding how they impact specific products is time-consuming, and simply hard to do effectively. Standard information ends up as siloed “tribal knowledge” known only to specific team members and lost with employee turnover.
Rimsys automates standards management, allowing RA teams to build customized libraries, get alerted when standards change, and proactively respond to prevent non-compliance or audit risks.
Medical device standards management software from Rimsys

Search our library of standards from over 200 organizations to find the ones you want to follow

Curate a library of standards that are relevant to your product portfolio, and share across teams

Link standards to individual products to easily assess the impact of changes across your product portfolio

Pull relevant standards information directly into your essential principles/GSPR tables for easier creation and maintenance
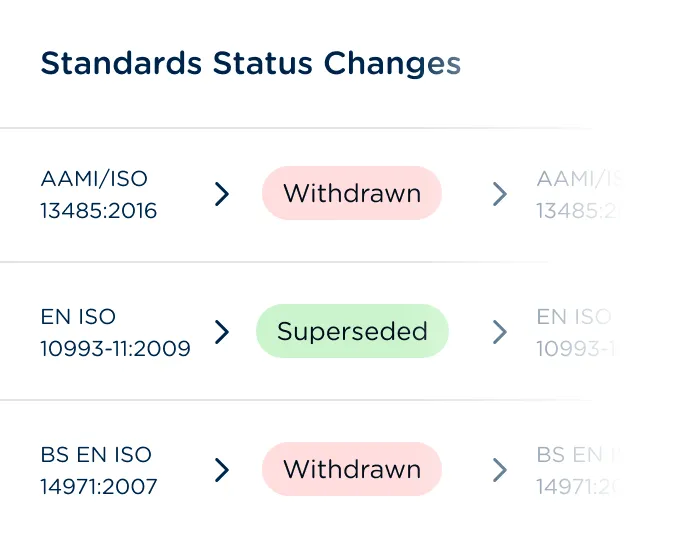
Get notified of changed, superseded, withdrawn, and work-in-progress standards
ISO 13485 overview - quality management requirements for medical device companies
ISO 13485:2016 defines quality management system (QMS) requirements for organizations producing medical devices. ISO 13485 compliance is required for most medical devices by all European Union members, UK, Canada, Japan, Australia, and many other countries.



