Digitally create, monitor, and manage product registrations and certificates. Stay updated on upcoming expirations and renewals with automated alerts.
Request a demo
With 113 different regulatory regimes impacting medical device sales around the world, it’s no wonder that 65% of regulatory professionals say that it takes a week or more to identify where their products are registered or sold. Managing device registrations with complex color-coded spreadsheets simply isn’t a viable option anymore.
Rimsys fully digitizes product registrations for easier management, and centralizes registrations, certificates, and supporting documentation at the individual product level, providing company-wide visibility into global market status for your product portfolio.
The result? You get complete visibility into global market status for your product portfolio, automated alerts on upcoming expirations and renewals, and strengthened compliance.

Track global registrations to keep ahead of expirations and other events that impact market status
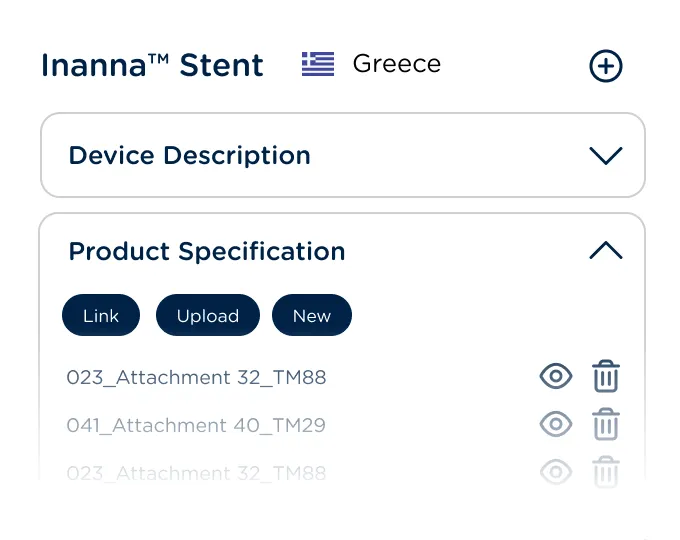
Manage requirements, content plans, documents, and tasks for new registrations

Integrate selling status with ERP and CRM systems to ensure global go-to-market alignment

Collaborate with distributors or other in-country representatives with controlled access to your registration information
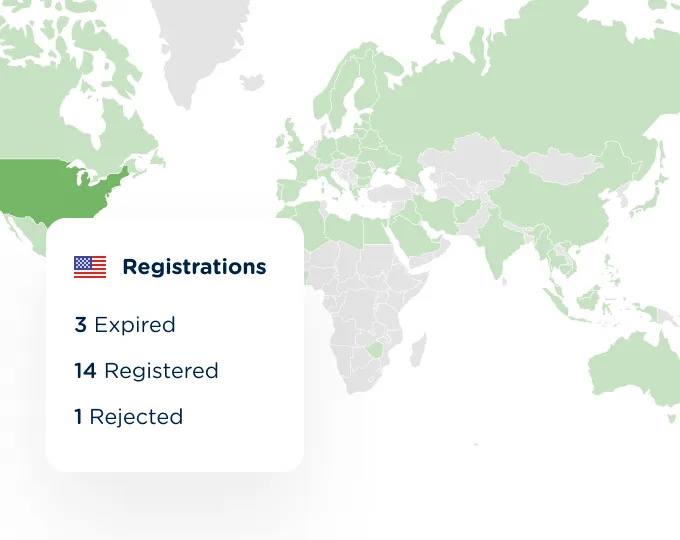
Click on any country and generate reports to see exactly where your products can be sold and track registration status
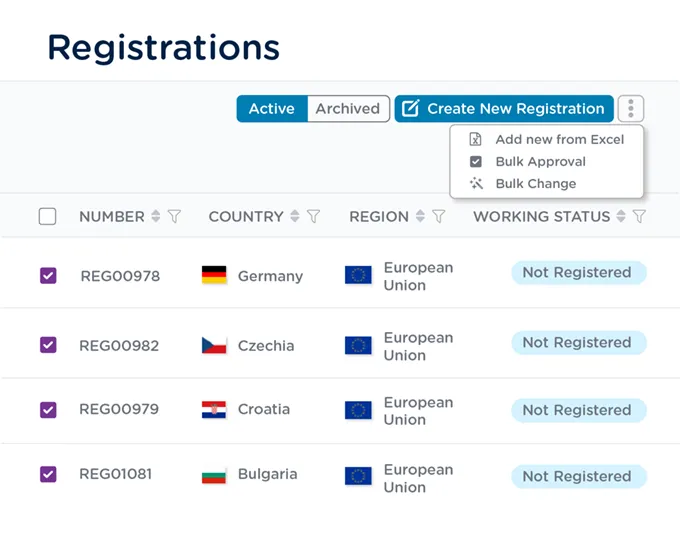
Route a group of registration records for approval in the same workflow and make changes to multiple records at once.
Like all products, time-to-market is a critical success factor for medical technology (MedTech). Unlike other products, however, medical devices have an added hurdle of regulatory clearance that must be obtained before products can be marketed and sold
