Integrated and automated global UDI management
Future proof your compliance with trusted UDI management designed to scale as global requirements change
Request a demo

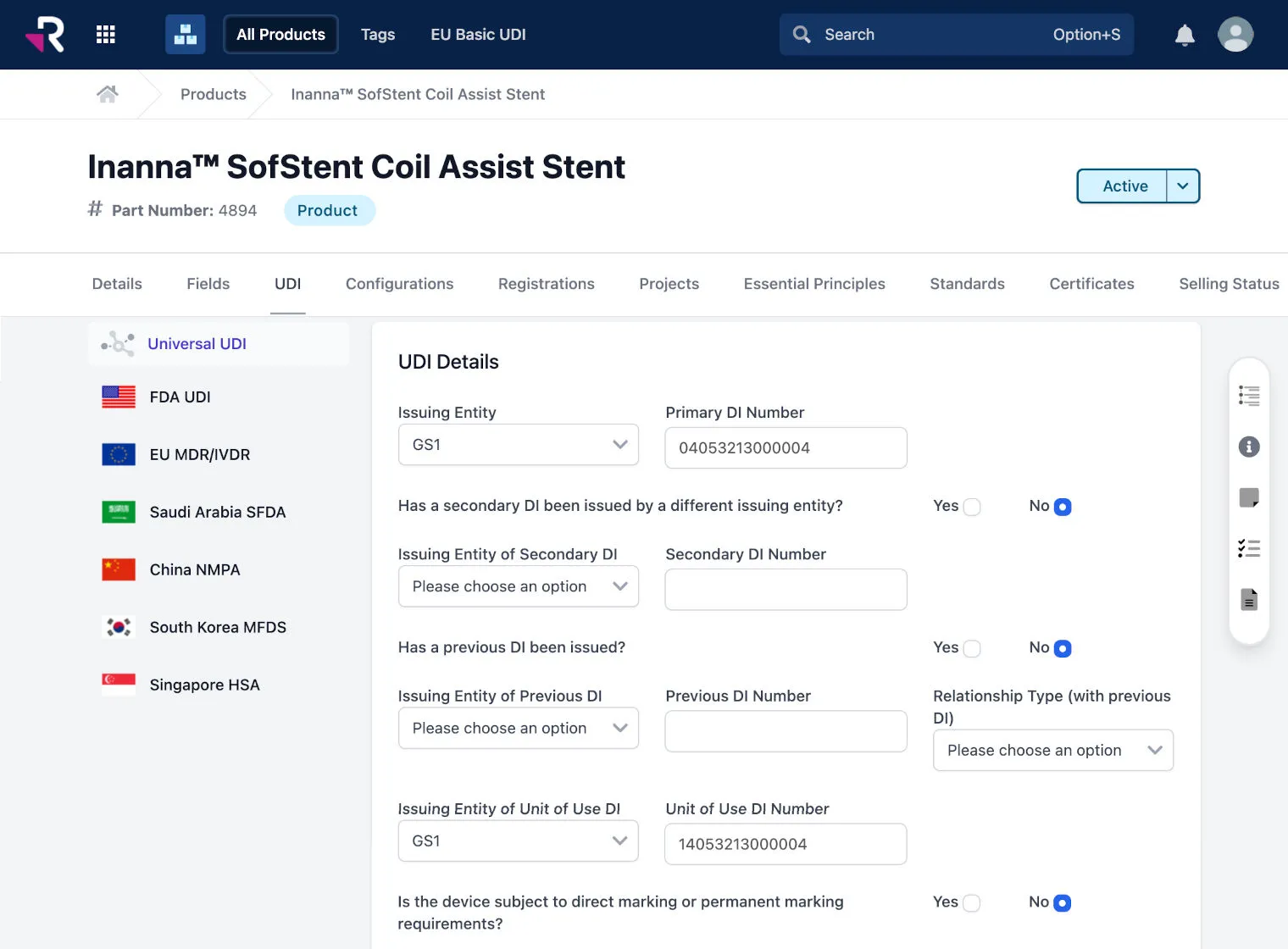
Associate and sync multi-country UDI data with product records for easy tracking and management
Reduce administrative burden and errors with Universal UDI®, which stores common UDI attributes and auto-generates country compliant formats for the US, EU, China, Korea, and more
Populate UDI data including expiration date, notified bodies, and classification from product and certificate records
Support EU MDR/IVDR requirements with category-level BUDI records, a complete EUDAMED data set, and transmission to EUDAMED (coming soon)
Submit UDI data directly to health authority databases to eliminate manual duplication and submission errors
Push UDI information from your PLM, ERP, CRM, and labeling systems to ensure consistency
The UDI requirements for EUDAMED are complex, but there is a tremendous opportunity: MedTech teams can position their EUDAMED data output as the foundation for a global UDI strategy. Learn more about the January 2026 UDI transmission requirements to EUDAMED and how Rimsys' novel Universal UDI® approach can set them up for long-term success as global UDI requirements change.
.webp)
Manage your UDI data alongside your regulatory information for total visibility and control

Rimsys organizes information at the individual product level, making it easy to see all of your associated regulatory activities in a unified RIM platform.


Manage your product registrations and certificates in Rimsys to see exactly where your products are registered and sold.


Leverage our API to easily push your UDI data from other systems such as your PLM and labeling software to Rimsys for seamless synchronicity.









